Research
Drug Discovery Research
Mochida Pharmaceutical's drug discovery research has made a fresh start with a major overhaul of its existing organization and strategy. In particular, we are focusing on "oligonucleotide drugs" to create innovative new drugs that address unmet medical needs.
Mochida Pharmaceutical Research Center located in Gotemba Shizuoka is mainly engaged in compound synthesis and biological evaluation, while the Pharmaceutical Laboratory in Fujieda Shizuoka conducts formulation studies and CMC research. In order to build a competitive research team, we are actively recruiting highly specialized personnel and strengthening our research infrastructure. In addition, Nissan Chemical Corporation  , which has excellent technological capabilities in oligonucleotide synthesis, will further accelerate research and the establishment of a manufacturing infrastructure. All of our researchers are working together to maximize the excellent properties of oligonucleotide drugs and to create original and unprecedented pharmaceuticals.
, which has excellent technological capabilities in oligonucleotide synthesis, will further accelerate research and the establishment of a manufacturing infrastructure. All of our researchers are working together to maximize the excellent properties of oligonucleotide drugs and to create original and unprecedented pharmaceuticals.
Oligonucleotide drug
Oligonucleotide drug is a new pharmaceutical modality that has been developing rapidly in recent years and is attracting a great deal of attention. The most important feature of oligonucleotide drugs is that they can target molecules that traditional drug modalities, such as small molecule drugs and antibody drugs, are unable to act on. Oligonucleotide drugs specifically bind to and degrade RNA molecules such as mRNA, thereby eliminating the protein molecules produced by the mRNA. Protein molecules that can be targeted by small molecule and antibody drugs are limited to those with specific structures. Therefore, it is believed that many useful protein molecules that could be targets for various diseases remain untapped as undruggable targets that cannot be targeted by small molecule or antibody drugs. Because oligonucleotide drugs can also target these molecules, they are expected to be able to create drugs for diseases that have been impossible to discover in the past.
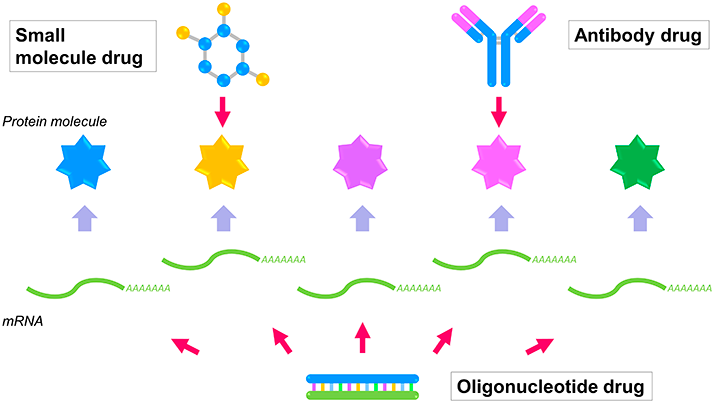
Small molecule and antibody drugs can act only on protein molecules with a specific structure (small molecule drugs: yellow protein molecules, antibody drugs: pink protein molecules). Oligonucleotide drugs can act on all types of mRNA (yellow-green), regardless of the structure of the protein molecules. Therefore, target molecules (blue, purple, and green protein molecules) that small molecule drugs and antibody drugs could not act on can be dealt with, and the possibility of target molecules (target space) can be expanded.
Open innovation
We are also actively engaged in open innovation in collaboration with various types of academia.
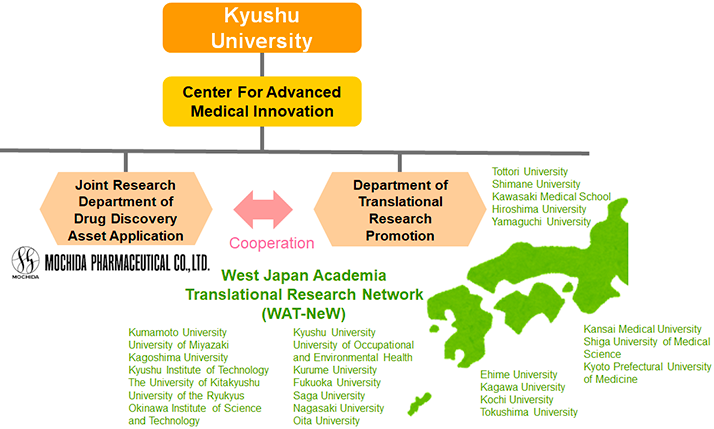
The "Joint Research Department of Drug Discovery Asset Application" has been established within the Center for Advanced Medical Innovation at Kyushu University to conduct collaborative research projects. In collaboration with the "Department of Translational Research Promotion", we access research information from the West Japan Academia Translational Research Network (WAT-NeW), which consists of 26 universities including Kyushu University, to collect promising drug seeds and drug discovery technologies.

MOIRe is a program that invites researchers in academia to submit promising drug seeds and technologies for drug development, both in Japan and abroad, and to collaborate with us in drug development. We are promoting collaborative research with researchers in academia in Japan and abroad on many of the ideas we have received so far.
Regenerative Medicine Products
In the field of regenerative medicine products, we are giving priority to projects using mesenchymal stem cells, and are currently in the process of developing therapies using stem cells from human exfoliated deciduous teeth (SHED), high purity mesenchymal stem cells (RECs: Rapidly Expanding Cells), and umbilical cord-derived mesenchymal stromal cells (HLC-001).
SHED
SHED are stem cells taken from the pulp cavity inside the tooth and are a type of mesenchymal stem cell. It is expected to be utilized in the future source of cells for regenerative medicine products that can be stably supplied domestically. We are working on the commercialization of regenerative medicine products with Kidswell Bio Corporation, which is an expert in human dental pulp-derived stem cells.
RECs
High purity mesenchymal stem cells or RECs (Rapidly Expanding Cells) are isolated from bone marrow aspirate and purified by a unique method established by PuREC Co., Ltd. These cells have superior proliferative, differentiation, and migratory capacities compared to mesenchymal stem cells isolated by conventional methods.
HLC-001
HLC-001 is a cell therapy product using mesenchymal stromal cells obtained from the umbilical cord, which is the tissue connecting the placenta and the fetus. We are working with Human Life CORD Japan Inc. which is using HLC-001 in research and development targeting multiple intractable disease on the commercialization of HLC-001.
Development Pipeline
| Development code | Generic name | Stage | Indications | Formulation | Remarks <Development country> |
|---|---|---|---|---|---|
| ACT-541468 | daridorexant | Filed | Insomnia | Oral | Co-development with Nxera Pharma Japan Co., Ltd. (Former Idorsia Pharmaceuticals Japan Ltd.) <Japan> |
| MD-711 | treprostinil | Filed | Pulmonary hypertension associated with interstitial lung disease or combined pulmonary fibrosis and emphysema | Inhalant | Licensed-in from United Therapeutics Corporation In-house development <Japan> |
| MD-0901 | mesalazine | Phase Ⅲ | Ulcerative colitis (pediatric indication) |
Oral | Licensed-in from Takeda Pharmaceuticals U.S.A., Inc. In-house development <Japan> |
| FYU-981 | dotinurad | Phase Ⅲ | Gout and hyperuricemia (pediatric indication) | Oral | Co-development with FUJI YAKUHIN Co., Ltd. <Japan> |
| MND-21 | icosapent | Phase Ⅲ | Hypertriglyceridemia | Oral | Collaboration with Sumitomo Pharma (Suzhou) Co., Ltd. <China> |
| Development code or Common name | Stage | Intended use or indications | Remarks <Development country> |
|---|---|---|---|
| dMD-001 | Filed | Articular cartilage lesion | In-house development <Japan> |
| dMD-002 | Therapeutic exploratory study | Cavernous nerve injury | In-house development <Japan> |
| dMD-003 | Therapeutic confirmatory study | Post-operative adhesion | In-house development <Japan> |
| Nerve Cuff | Filed(510(k)) | Peripheral nerve injury | In-house development <USA> |
